“They sacrificed their sons and their daughters unto devils, and shed innocent blood, even the blood of their sons and of their daughters, whom they offered unto the idols of Canaan, and the land was defiled with blood.”
Psalm 106:37-38
When, in December 2020, Pfizer’s COVID ‘vaccine’ gained emergency-use authorization (EUA) for adults, the company had apparently won its ‘Warp-Speed’ sprint to develop a countermeasure against the dreaded SARS-CoV-2 virus.
But as it turned out, this was just the first leg in a race to inject younger and younger age groups. Just five months later, on May 10, 2021, the FDA extended Pfizer’s EUA down to 12‑year‑olds, then to five-year-olds on Oct. 29, 2021, and finally to six-month olds on June 17, 2022.
Even that did not satisfy Pfizer, as we now know from its document titled License Action Recommendation Documents for Submissions and FDA Communication. It shows that, in July 2021, Pfizer was in talks with the FDA about “another study to enroll infants <6 months of age” (p.10).
Indirect Harms to Children
Pfizer didn’t entirely get its way, though, because the EUAs stopped at six-month-olds, but it managed to target even younger victims anyway, either in the womb or at the breast of their injected mothers. We know this from Pfizer’s Cumulative Analysis document that tallied worldwide adverse-event reports in the opening months of vaccine authorizations. Like the aforementioned License Action document, it is part of Pfizer’s biological product file extracted from the FDA through sustained legal action.
… In the womb.
Based on reports Pfizer received from 63 countries between December 2020 and the close of February 2021, the Cumulative Analysis document tallied 270 pregnancy cases, representing 271 potential babies because one involved twins. Pfizer did not know the outcome for 238 pregnancies, and a further five pregnancies were “outcome pending.”
That meant Pfizer knew the outcomes for only 27 pregnancies (270 minus 238 minus 5), representing 28 potential babies, including the one set of twins. This is where the tragedy hits hard, because 26 of those 27 pregnancies experienced “spontaneous abortion,” and there was one instance of “premature birth with neonatal death.”
THUS, 27 OF THE 28 POTENTIAL BABIES DIED. THERE WAS BUT ONE SURVIVOR, LISTED AS “NORMAL OUTCOME.”1
These numbers, devastating as they are, represent but a sample of the total because, as the document notes, “Reports are submitted voluntarily, and the magnitude of underreporting is unknown” (p.5).
The same applies to Pfizer’s Periodic Safety Update Report (PSUR #1), spanning 286 pages, to the European Medicines Agency (EMA). Covering the six‑month period from Dec. 19, 2020 through June 18, 2021, this ‘pharmacovigilance’ document is supposed to detect safety signals based on adverse-event data both from Pfizer’s clinical trials and from its post-authorization rollouts. These are listed separately. The report was not publicly released until early 2023 after an unnamed researcher obtained it via FOIA request and provided it to TKP, an Austrian blog.
PSUR #1 records 1,604 pregnant-mother cases in its post-authorization data, of which 945 led to adverse events. The serious events with nine occurrences or more were: “Abortion spontaneous (275), Vaginal haemorrhage (27), Abortion missed (21), Foetal death (16), Abortion (9)” (p.241). In addition, there were seven elective pregnancy terminations, of which six were due to “foetal defects” (p.242).
Pfizer’s third Periodic Safety Update Report (PSUR #3) to the EMA, covering the year-later period (Dec. 19, 2021 to June 18, 2022) added a further 566 spontaneous‑abortions from 1,898 known pregnancy outcomes, and 78 cases of vaginal haemorrhage (p.336). There were 23 elective pregnancy terminations, 22 of which were “due to foetal defects,” with the reason for the one remaining termination unknown (p.337).
Among the infants born alive, 31 infants were born with congenital anomalies, including cardiac disorder, chromosome abnormality, growth restriction, kidney malformation, congenital amputation, and congenital disorders of foot, hand, limbs, and spine.
At the breast.
Even then, babies were not safe from indirect vaccine injury, as those who survived ‘transplacental’ harms were then in danger of ‘transmammary’ harms through breast milk that contained vaccine products from injected mothers. There were early warning signs of this in Pfizer’s Cumulative Analysis document, which noted one case of suppressed lactation and one of breast-milk discoloration.
Shortly after, reports of infant deaths from tainted breast milk started appearing in the Vaccine Adverse Events Reporting System (VAERS), a database jointly managed by the FDA and the CDC. The earliest reports include…
A breast-feeding five-month-old boy who died soon after his mother got a second Pfizer dose on March 17, 2021. The next day, he “developed a rash and within 24 hours was inconsolable, refusing to eat, and developed a fever. Patient brought baby to local ER where assessments were performed, blood analysis revealed elevated liver enzymes. Infant was hospitalized but continued to decline and passed away. Diagnosis of TTP. No known allergies. No new exposures aside from the mother’s vaccination the previous day” (VAERS ID: 1166062).
A one-year-old boy who went into intense febrile seizures on Feb. 19, 2021 after “vaccine exposure via breast milk.” His mother had received a first Pfizer dose four days earlier (VAERS ID: 1161763).
A six-week-old boy who died on July 17, 2021 “from clots in his severely inflamed arteries,” as reported to VAERS by his 36-year-old mother. “I had been breastfeeding my 6 week old baby at the time that I received the first Pfizer vaccine… I am curious if the spike protein could have gone through the breast milk and caused an inflammatory response in my child” (VAERS ID: 1532154).
Many more such cases are cited in a NewsRescue article of Sept. 22, 2021. It also notes, “A number of reports describe mothers’ milk drying up suddenly after vaccination.”
Transmammary harms snowballed further in Pfizer’s PSURs to the EMA. The cases are harder to tease out from these large documents, but they include 61 cases deemed ‘serious’ in PSUR #1 (p.245) and a further 66 serious cases in PSUR #3 (p.340). Elsewhere, the documents list two cases of stroke among breastfeeding infants (PSUR #1, p.163), three neurological cases (PSUR #1, p.149), and four respiratory cases (PSUR #3, p.239).
That vaccine contents make their way to breast milk was also demonstrated in a study published September 2022 in JAMA Pediatrics. It found that, among 11 lactating women, trace amounts of Pfizer and Moderna vaccine contents were detected in the breast milk of five women within 45 hours of receiving a vaccination (Table 2).
So what did Pfizer conclude after such a litany of harm in the PSURs? That “There were no safety signals regarding use in pregnant/lactating women that emerged from the review of these cases or the medical literature” (PSUR #3, p.340). Naturally, the various regulatory authorities rubber-stamped Pfizer’s self-serving analysis, and the public were none the wiser.
How did Pfizer achieve this sleight of hand? Sometimes, it would cite prior medical conditions in the mother, or her lifestyle choices, as contributing factors in harms to mother or child; occasionally, it cited the mother’s advanced age for childbearing; but most of the time, Pfizer resorted to “limited information regarding the mother’s obstetric history, which precluded meaningful causality assessment” (e.g., PSUR #3, p.331).
Shedding parents.
Yet another vector of danger for young children is to receive secondhand vaccine contents from jabbed parents, whether via inhalation or skin contact. Since 2001, microscopy analysis has shown disturbing structures in blood samples taken from the injected, but then similar structures started appearing in the uninjected who had been in close proximity to jab recipients. These effects have proven especially harmful in children.
Dr. Philippe van Welbergen, a U.K.-based physician, has been foremost in sounding the alarm. In a February 2022 presentation, he showed a blood sample from an unjabbed eight-year-old child who became partially paralyzed after their blood was devastated by transmitted injection components (around 1:14:30).
Direct Harms to Children
And what of children given the needle itself? The Cumulative Analysis report alone gave early and ample warning of what lay ahead. No child was supposed to receive a Pfizer shot during the reporting period, which ended Feb. 28, 2021, but Pfizer knew of 34 children aged between two months and nine years who did. Twenty-nine of them were in the U.K. Of the 34 children, 24 experienced at least one serious adverse event (p.13).
Table 7 of the report also details two childhood-injury cases reported to Pfizer by the U.K. Medicines and Healthcare products Regulatory Agency (MHRA, p.25). The first “described a 1-year-old subject who received the vaccine, and had left postauricular ear pain that progressed to left-sided Bell’s palsy 1 day following vaccination that had not resolved at the time of the report.” Another “described a 7‑year‑old female subject who received the vaccine and had stroke (unknown outcome); no follow-up is possible for clarification.”
Yet Pfizer’s paper concludes, “No new significant safety information was identified based on a review of these cases compared with the non-paediatric population” (p.13). Should we be comforted by this language, telling us the outcomes for children were no worse than the outcomes for adults? Not when the document counted 1,223 post‑injection deaths among all age groups (p.7) among 42,086 individuals who experienced between them 158,893 events.
Then the document’s appendix (p.30) lists 1,294 adverse events of special interest (AESI), meaning pre-specified medically-significant events that could be caused by the vaccine product. These include ‘Child-Pugh-Turcotte score abnormal’ (signalling liver disease), ‘Convulsion in Childhood’, ‘Multisystem inflammatory syndrome in children’, and ‘Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection’.
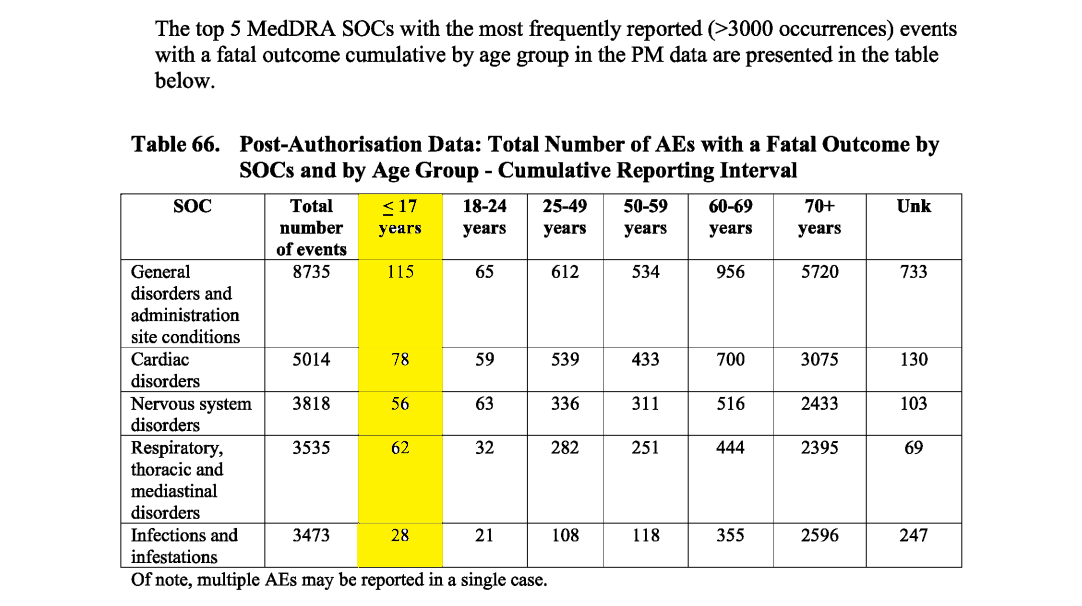
Again, Pfizer’s PSURs to the EMA darken the horrors still further, with PSUR #3 logging 161 post-authorization fatalities among under-18s in the cumulative data set (combining all PSURs to date) (p.292). These deaths were tied to 339 adverse events (meaning there could be multiple adverse events for one individual), with 78 categorized as cardiac disorders, 56 as nervous-system disorders, and 62 as respiratory, thoracic, and mediastinal (p.293).
Five- through 11-year-olds.
PSUR #3 then segments the pediatric data by age group. It records 20 post-authorization deaths among 9,605 injured individuals aged five through 11 years old in its six-month reporting period (p.321).
One death involved an 11-year-old boy who went into acute respiratory failure two days after his first dose of Pfizer’s ‘BNT162b2’ vaccine. No autopsy was performed. Another was a six-year-old girl whose fatal adverse events included kidney impairment and seizure. She died seven days after her first dose. It was unknown whether an autopsy was performed. A six-year-old boy died with myocarditis and cardio-respiratory arrest seven days after his first dose, though “the reporter concluded that the death ‘had nothing to do’ with the administration of BNT162b2 and was due to natural causes.” Autopsy results were pending.
Among the remaining fatal cases, cardiac and respiratory events were the main killers. Cerebral haemorrhage, multisystem inflammatory syndrome, and pulmonary embolism were also among the reported events (p.322).
12- through 17-year-olds.
Among 12- through 17-year-olds, PSUR #3 tallies 62 fatal cases post authorization in its six-month reporting interval. Here, Pfizer made a very rare concession to possible causation, though only for 20 of the 62 deaths, for which “a causality between the vaccination and the occurrence of the fatalities cannot be ruled out, based on the temporal relationship, although no laboratory data or autopsy results provided evidence of a causal relationship.”
Pfizer provided more detail on six of the deaths where it cited “underlying medical conditions” as a possible factor. A 16-year-old girl died of dyspnea three days after first dose, not known if autopsy performed. Another 16-year-old girl experienced brain injury and acute cardiac failure six days after first dose. She died suddenly of cardiac failure 35 days later, no autopsy performed. A third 16‑year‑old girl died of pulmonary embolism and cardiac arrest after third dose, autopsy performed but results not provided. A 13-year-old girl experienced chest pain and palpitations five days after first dose. She went into cardiac arrest and lost consciousness two months later. An autopsy was performed and determined that her death was possibly related to the vaccine.
A 17-year-old boy died of cardiac failure 92 days after second dose. The autopsy results were heavily redacted. And a 13-year-old boy died of brain death within one month of unknown dose number, not known if autopsy performed.
PSUR #3 also gives insight into the psychological toll inflicted by Pfizer’s medicine, with two suicide attempts among 15 individuals harmed in the 12-17 age group during clinical trials within the six-month period (p.323).
Yet Pfizer concluded after all this, ”No new significant safety information was identified based on the review of the cases reported in the overall paediatric population” (p.328).
Myocarditis.
Bear in mind that the heart events described in PSUR #3, many of them fatal, occurred some six months or more after the CDC’s Advisory Committee on Immunization Practices (ACIP) acknowledged “reports of myocarditis and pericarditis in mRNA vaccine recipients” in a July 2021 update, but it continued to recommend “that all persons aged ≥12 years receive a COVID-19 vaccine.” Its benefits, the committee maintained, “clearly outweigh the risks in all populations, including adolescents and young adults.”
In June 2022, Journal of Pediatrics published a study from Seattle Children’s Hospital reporting that over a nine-month period, 35 patients younger than 18 years of age presented with chest pain within one week of receiving a second Pfizer dose. All were diagnosed with myopericarditis, meaning inflammation of both the heart muscle and its lining.
The report presented findings on 16 of those patients who had both acute-phase and follow-up cardiac MRIs available for review. There were 15 boys in the group and one girl, and the median age was 15 years. Median time to presentation after the second Pfizer dose was three days. All patients had elevated serum troponin levels, indicating “increased risk of heart attack,” and all initial cardiac MRIs were abnormal.
In follow-up MRIs, conducted between three and eight months later, 11 of the 16 patients had persistent late gadolinium enhancement (LGE), signifying ongoing myocarditis and could be “predictive of a poor outcome.” LGE, the paper went on to explain, “is an indicator of cardiac injury and fibrosis… Yang et al found that presence of LGE is a predictor of all-cause death, cardiovascular death, cardiac transplant, rehospitalization, recurrent acute myocarditis, and requirement for mechanical circulatory support.”
Seizures.
Another journal, Pediatrics, published a study in June 2023 titled Safety of COVID-19 mRNA Vaccination Among Young Children in the Vaccine Safety Datalink, where Vaccine Safety Datalink is a collaboration between CDC and eight data-contributing health systems in the U.S. From 135,005 Pfizer doses given to children aged six months to four years, the study reported 38 seizure events within three weeks of any dose and a further 24 seizure events in weeks four through six after most recent dose (Table 2).2
The authors rejoiced, however, that they “did not detect a safety signal for any outcome during the 21 days after vaccination” while omitting days 22-42 in this assessment. These results, they concluded, “can provide reassurance to clinicians, parents, and policymakers alike.”
Maddie de Garay
Horrifying as all these numbers are, they do not describe the heartbreak of parents who would rue the day they brought their loved ones in to get an experimental injection. Among those parents are Stephanie and Patrick de Garay. Their daughter, Maddie, was a healthy 12-year-old girl until, on Jan. 20, 2021, she received a second Pfizer dose in clinical trials for adolescents at Cincinnati Children’s Hospital.
About 18 hours after, Maddie developed severe nerve pain, a feeling like electric shocks going down her neck and spine, excruciating abdominal pain, and severe chest pain that felt like her heart was being pulled out. Her vaccine arm swelled up and became numb. Her fingers and toes turned white, and were ice cold to the touch. The pain in her toes was so bad that she had to walk on her heels.
Stephanie and Patrick took Maddie to the ER at Cincinnati Children’s Hospital where they screened her for appendicitis and ruled it out. Though they found dilated loops of bowel on X‑ray, and observed blood in her urine, they sent her home.
Over the following months, Maddie’s condition worsened, including food regurgitation and vomiting, until she was unable to swallow any food or liquids, and had to resort to a feeding tube. The total body pain and severe abdominal pain persisted, and her abdomen became distended. She experienced fainting spells which developed into seizures, sometimes 10 a day.
Prior to injection, Maddie had never menstruated, but on Feb. 5, 2021, she started to discharge brown fluid, which came out in chunks over the following month. Painful cysts developed on her vagina and then her head. Erratic blood pressure and heart rate were also among the symptoms, along with tinnitus, vision problems, headaches, dizziness, and memory loss. Finally, she lost feeling from the waist down, which led to paralysis and incontinence.
Maddie went to the ER eight more times as her health continued to decline, until she was admitted to Inpatient Rehabilitation at Cincinnati Children’s Hospital on Apr. 13, 2021. There she remained for several weeks through her 13th birthday.
A dysfunctional diagnosis.
By now, Maddie was traumatized by her experiences with doctors, who refused to believe her or acknowledge the injection triggered her symptoms. They treated her like a mental patient instead, using the facile diagnosis of ‘functional neurological disorder’. After that, they stopped any further testing.
Any diagnosis that includes the word ‘functional’ invites skepticism. As observed by Jessica Wallace of law firm, Siri & Glimstad, an attorney with a background in vaccine-injury cases and medical malpractice, “This is a diagnosis of exclusion, and basically, they are saying it’s all in the patient’s head.” The term also infects future diagnoses and treatments by encouraging doctors to write off patients and their symptoms with mental or behavioral labels.
One glaring example of the medical indifference Maddie endured came from Amal Hallim Assa’ad, M.D. at Cincinnati Children’s. She conducted just one 15‑minute consultation with Maddie on Mar. 5, 2021, but deemed this sufficient to rule out further tests. “It drives the patient to thinking that there must be something wrong that is indicating all this workup,” Assa’ad wrote in her notes “It also delays the necessary psychological intervention that is needed to help resolve the functional disorder.” The hospital then tried to discharge Maddie to a facility for eating disorders.
Furthermore, the misdiagnosis was formalized as ‘abdominal pain’ in the safety-evaluation data Pfizer turned over to the FDA as it sought authorization for its prized serum. Even that one symptom was viciously understated, as Maddie would scream in agony from that one symptom alone. On Feb. 28, 2021, from an adjoining room, Stephanie recorded her daughter crying out, “I can’t do this. I can’t do it. I’m gonna die. God, why are you doing this?!”
But then there was more insult added to Maddie’s injury as Pfizer refused to cover her staggering medical bills. This devastating news came in a May 17, 2021 phone conversation with Dr. Robert Frenck, principal investigator for the clinical trial. The costs would not be covered, he told Patrick and Stephanie, because they were not deemed “research related”—in other words, not attributable to the trial. “The doctors that have seen her so far have not found something where they thought it was research related,” he said, “is what they were all telling me.”
Patrick was astonished. “Within a 24-hour period, all this went down,” he said, “so if that’s your stand, I find it hard to believe that you’re trying to steer away from that.” He tried to restrain himself as he continued, “Don’t worry about my daughter can’t walk or anything. As you can tell, Dr. Frenck, I’m a little upset.”
Nevertheless, Frenck continued to insist the costs would be the family’s responsibility, a clear and egregious breach of international law, specifically the Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Para. 15 states, “Appropriate compensation and treatment for subjects who are harmed as a result of participating in research must be ensured.”
But Frenck was not done. Just 10 days later, he appeared as lead author on a New England Journal of Medicine article, published May 27, 2021, reporting on Pfizer’s vaccine trial for adolescents, in which Maddie participated. Pfizer’s vaccine “had a favorable safety and side-effect profile,” he asserted. “There were no vaccine-related serious adverse events and few overall severe adverse events.”
Pfizer’s whitewash shows up in PSUR #1, in the section showing clinical-trial data for children aged 12 to 15. It records that 14 children experienced adverse events, with one occurrence of ‘Abdominal pain’. The full list comprises “Depression (4), Suicidal ideation (3), Appendicitis (2), Abdominal pain, Anal abscess, Anxiety, Constipation, Conversion disorder, Femur fracture and Focal peritonitis (1 each). All events were assessed as unrelated to BNT162b2 or blinded therapy” (p.228).
Rogue Regulators
So how did regulators respond to all of this? The FDA had in its possession Pfizer’s Cumulative Analysis report by May 2021 at the latest, as Pfizer’s letter enclosing it is dated May 6, 2021. Its tidings of 1,223 deaths were reason enough to pull Pfizer’s vile vials from every hospital, clinic, care home, and pharmacy in the world. In the words of renowned cardiologist and epidemiologist, Dr. Peter McCullough, in a Dec. 7, 2021 interview with DarkHorse Podcast, “Our vaccine program would have been shut down in January [2021] for excess mortality.”
Yet the FDA’s damage control was not to protect the lives of children but to protect the image of vaccines. Thus, Peter Marks, director of the FDA’s Center for Biologics Evaluation and Review (CBER), told a June 29, 2021 FDA ’stakeholder call’ that child deaths post-vaccination had been “very sensationalized” and were attributable to a “definitively different cause of death.” He admitted that post-vaccine myocarditis was “about five times higher than the baseline” in males aged 12 to 17, but these cases had “generally been mild.”
Adolescents were not going into heart failure, Marks said, but “may have a twinge of chest discomfort.” Vaccines could trigger “a somewhat exuberant immune response… that causes a little bit of inflammation in the heart. In this case, it appears to be relatively brief. Again, goes away on its own.”
Mark’s presentation estimated COVID vaccines led to 56-69 cases of myocarditis per million males aged 12 to 17, but he deemed this a fair price to pay for the two COVID deaths he said were prevented by injecting a million boys. He had “no hesitation whatsoever about this recommendation,” even for those with pre-existing heart conditions. “Getting your child vaccinated now is something that is going to be good to do,” he added, and two shots were better than one because “generally, the immunity after natural infection tends to wane after about 90 days. And we do know that the immunity after vaccination is better than the immunity after natural infection.”
More perception management would be required, however, as the FDA extended Pfizer’s gift (at least in the German sense of the word) to younger and younger children. On Apr. 26, 2022, Marks appeared in an FDA video—one of many in a nauseating series titled Just a Minute!—to promote COVID jabs for kids under five years of age. It was very troubling, he said, that only 27% of children five through 11 had been vaccinated against COVID, but he assured parents the FDA would be “thorough and transparent in our evaluations… without sacrificing our standards.”
The outcome of these evaluations, however, was a foregone conclusion. Marks continued, “Once our reviews have been completed, we will bring the vaccines before our independent advisory committee to have a transparent discussion of the data, so that when we make a determination to authorize the vaccines in young children, parents can be confident in their decision to vaccinate.”
On Apr. 29, just three days later, Marks followed up with another Just a Minute! in which he promised the FDA would “not cut any corners” in evaluating shots for the under-fives. He didn’t have to wait long because, as we have seen, the FDA extended its EUA to six-month-olds for both Pfizer and Moderna on June 17, 2022. At the following press conference, Marks asserted that the vaccines were “safe and effective for use in our nation’s youngest children.”
Reading from a teleprompter, he continued, “The FDA was acutely aware of the trust bestowed on it by the American people.” Parents, caregivers, and health-care providers could “trust that both of these vaccines have been authorized with science and safety at the forefront of our minds.” He closed by urging “individuals of all ages to get vaccinated and boosted when eligible if you’ve not done so already.”
The following day, Rochelle Walensky, then director of the CDC, dutifully applauded in a tweet, “We now know based on rigorous scientific review that the vaccines available here in the United States can be used safely and effectively in children under 5.”
Last throw of the dice: ‘bivalent’ boosters.
Even so, Pfizer’s race had one more lap to go. Just 11 days after the FDA extended EUAs to six-month-olds, the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) met on June 28, 2022 to discuss ‘bivalent’ COVID shots, which purportedly addressed more than one virus ‘variant’.
Children were in the crosshairs yet again. Dr. Mark Sawyer, for example, said he didn’t want the rollout to be “too late” to address a predicted wave of new COVID infections in the Fall. Though he acknowledged “concern about side-effects and serious side-effects in children,” he urged, “We are unlikely to learn about those during any clinical trials, so I think—myocarditis in particular—we’re only going to know that when we roll out the vaccine, and the safety systems do their review” (around 8:09:00).
Sawyer was then echoed by Dr. Amanda Cohen who called for haste in future authorizations for children under five, saying, “I don’t want concerns about myocarditis to increase the amount of time it takes to get a vaccine available for this age group” (at 8:10:10).
No surprise, then, that on Aug. 31, 2022, about a week after receiving requests from Pfizer and Moderna, the FDA authorized Pfizer’s new ‘updated boosters’ for individuals 12 years old and up, and Moderna’s for 18 and up. Both bivalent brews were later authorized to six-month-olds on Apr. 18, 2023, just in time for the official “End of COVID-19 Public Health Emergency” on May 11, 2023.
Conclusion
What motive, then, shall we attribute to Big Pharma’s ruthless campaign of violence against children? According to investigative reporter, Lara Logan, the assault is as much spiritual as physical. “For them, the younger you are, the closer you are to God, the more pain they can inflict on God,” she explained in a June 2022 interview with Vigilant Fox. “So the more you can make a baby or a small child suffer, the greater your victory over God. And that is the only consideration for them.”
From this perspective, Pfizer is not just a corrupt corporation counting the dollars but a rapacious priesthood counting the bodies. The younger its sacrificial victims, the greater its prestige before medical deities. What else can explain its wanton slaughter of children, not just in the cradle, but in the womb and at the breast, and all at industrial scale. If, as the Bible warns, “The wages of sin are death,” how much more so when the sins are against the child?
Abdiel LeRoy is the author of some 30 books. Find The REAL Pfizer Papers: What Investigators Missed in the Documents Released by the FDA, at https://Geni.us/Pfizer.
_______________________________________________________________________
1 This calculation is derived from the following text in Pfizer’s document: “Pregnancy outcomes for the 270 pregnancies were reported as spontaneous abortion (23), outcome pending (5), premature birth with neonatal death, spontaneous abortion with intrauterine death (2 each), spontaneous abortion with neonatal death, and normal outcome (1 each). No outcome was provided for 238 pregnancies (note that 2 different outcomes were reported for each twin, and both were counted).”
2 From 112,006 Moderna doses, the study recorded 23 seizure events within three weeks of injection, and a further 19 seizure events in weeks four through six, for children aged six months to five years.